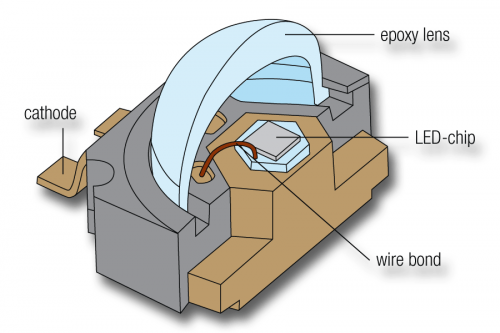
Whereas in conventional lamps light is emitted by a filament or a gas, LEDs are tiny electronic chips made from special-purpose semiconductor material. If electricity flows through the chip it starts to emit light (a process known as electroluminescence). LEDs act very nearly as a point source of light, without radiating heat.
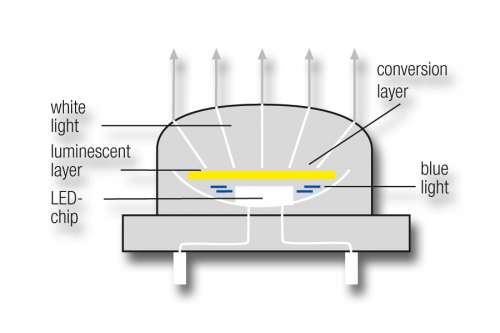
These diodes can emit only single-coloured light, which must then be converted into white light. The currently most satisfactory method uses the principle of colour conversion by luminescence: in this case an ultra-thin layer of yellow phosphorus, brought up over a blue LED chip, converts some of the blue light into white light. A second way of obtaining white light from LEDs is to mix coloured light of differing wavelengths.
LED modules have an extremely long service life (more than 50,000 hours of operation) and are remarkably energy-efficient. LEDs deliver the same amount of light (measured in lumen) as conventional lamps, but consume much less electricity. For instance, a 60-watt bulb delivers about 600 lumen, while an LED lamp needs a mere 8 watts to deliver 600 lumen.
As the starting-point for LEDs, semiconductor crystals are grown on a substrate and then sliced into chips. The diodes feature a negatively conducting base semiconductor with an electron surplus. An ultra-thin positively conducting semiconductor layer with “holes”, i.e. a shortage of electrons, is deposited on this base. When a voltage is applied, the surplus electrons and the holes recombine in the so-called depletion layer of the semiconductor crystal, releasing energy in form of light.
Share